BrainsWay's Deep TMS
BrainsWay’s Deep Transcranial Magnetic Stimulation (Deep TMS™) is a unique, non-invasive treatment that utilizes magnetic fields to treat various mental health conditions, by effectively regulating the neural activity of brain structures found to be associated with those conditions. FDA-cleared to treat both depression and OCD, Deep TMS has been shown to be well-tolerated and proven effective in alleviating adverse symptoms.
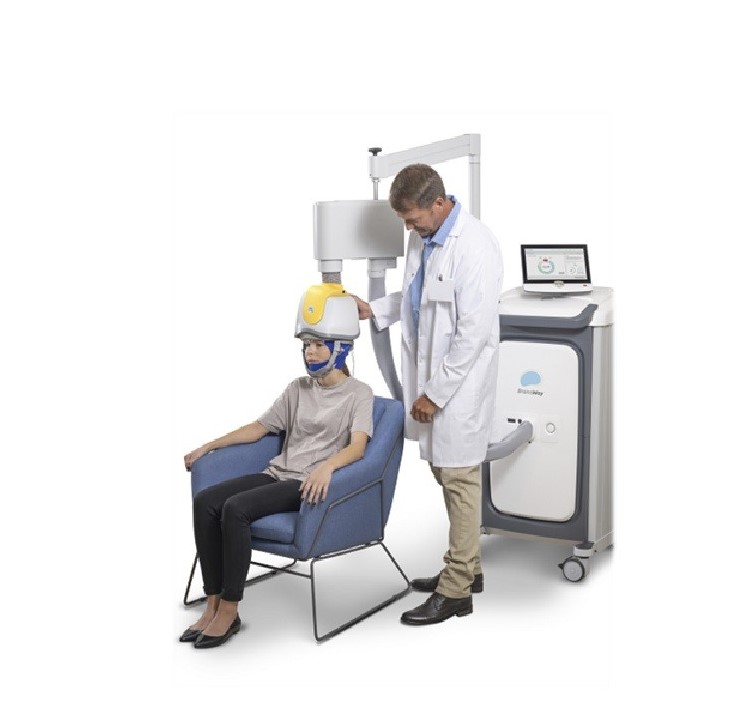
During the Deep TMS treatment process, a padded helmet containing BrainsWay’s patented H-coil is fitted over the patient’s head. The helmet then sends out magnetic pulses that influence the neural activity of the relevant brain structures. The non-invasive treatment process does not require anaesthesia, and each session lasts about 20 minutes, allowing you to incorporate them into your daily routine.
Proven to Be Effective
A growing body of research has found Deep TMS to successfully alleviate symptoms of mental health conditions. These studies use gold- standard benchmarks, such as randomized, double-blind protocols and in-depth analyses, to reach solid, evidence-based conclusions. They have been published in such well-regarded journals as The American Journal of Psychiatry, World Psychiatry and the Journal of Psychiatric Research.
Due to its success in offering symptom relief and its favourable safety profile, Deep TMS has already been granted FDA clearance to treat both major depressive disorder (MDD) and obsessive- compulsive disorder (OCD).
Read More about BrainsWay Deep TMS Treatments
A 2015 study published in World Psychiatry highlighted Deep TMS’s effect on treatment-resistant participants with MDD. The study, aggregating data from 20 academic medical centres, concluded that Deep TMS treatment led to remission in roughly one in three participants following the acute phase’s four weeks of treatment.
Study participants were treatment-resistant patients who had previously not significantly benefited from antidepressant medication. Results from this study were considered so conclusive as to earn Deep TMS its first FDA clearance status.

Deep TMS has been shown to be even more effective in real-life clinical settings – often as an adjunct form of therapy together with other treatments, such as antidepressant medication and psychotherapy. Based on the recorded data of over 1000 participants undergoing Deep TMS treatment for MDD, 75% achieved a clinical response, with one in two achieving remissions.

In addition to depression, Deep TMS has also been FDA-cleared (De Novo) to treat obsessive-compulsive disorder (OCD). Its efficacy in treating this condition was demonstrated in a 2019 publication in the American Journal of Psychiatry. The study utilized the Y-BOCS rating scale, considered the gold standard tool used by psychologists and psychiatrists to measure the severity of OCD.
A “response rate” was defined as a reduction of at least 30% of the subjects’ symptoms after undergoing Deep TMS therapy, and a “partial response rate” was defined as a reduction of at least 20%. The study found that Deep TMS treatment offers significant clinical improvement for treatment-resistant patients, with more than half of patients achieving a partial response, and about a third of patients achieving a response.

The study demonstrated efficacy even among patients who had previously been found unresponsive to either psychotherapy or medication—which make up a large percentage of those battling OCD. Furthermore, these patients’ improvement was sustained for several weeks following the treatment’s conclusion.
Click this link to access Clinical Research, Webinars, Testimonials and other resources.
Read the Patients' Stories
Download the following patient brochures.

