Management of Major Depressive Disorder with a Focus on Pharmacogenetics | Part Three: The “Other Antidepressants”
Did you know that the first “antidepressant” was discovered by accident? The discovery of iproniazid and its mechanism of action led to the discovery of the monoamine hypothesis and paved the way for the antidepressant boom. Tweaking chemical structures to alter mechanisms of action led to safer versions of drugs like iproniazid and the creation of drug classes like the tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs).
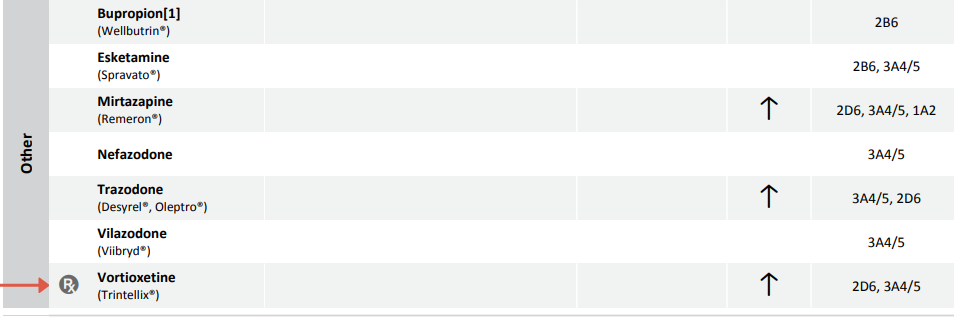
In the last few decades, several more antidepressants with unique mechanisms of action have been developed. These medications are listed under the class of “Other Antidepressants” on the Gene Drug Interaction Summary in the Genomind® Professional PGx Express™.
DWhile most of the pharmacogenetic (PGx) research and published guidelines in psychiatry have focused on the SSRIs, SNRIs, and TCAs, there is growing evidence that PGx can provide useful information for other medications that are approved for use in major depressive disorder (MDD).
Pharmacogenetic Considerations of Bupropion (Wellbutrin)
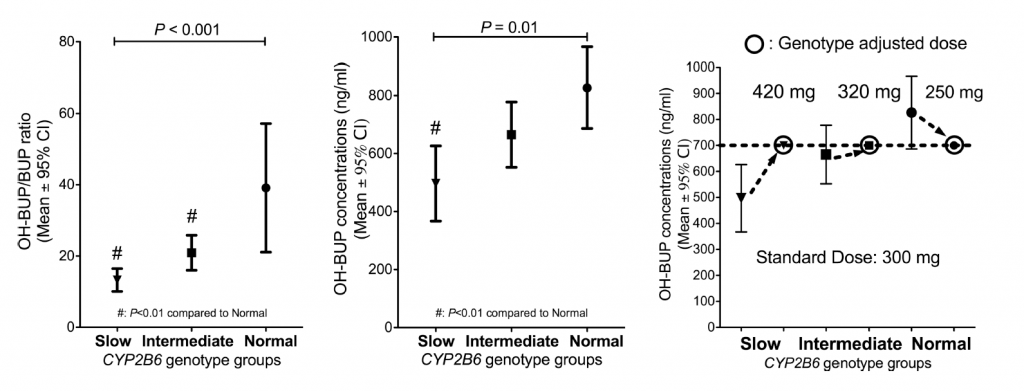
Bupropion is a substrate of CYP2B6, and polymorphisms within this gene have been shown to alter the level of bupropion and the active metabolite hydroxybupropion. One study by Zhu and colleagues found that patients (n = 154) with genetic variants that slowed the metabolism of bupropion produced less of the active metabolite, and required higher doses in order to achieve efficacy in a study of smoking cessation. As shown in Figure 1, slow and intermediate metabolism were associated with lower levels of hydroxybupropion. This study and others suggest that bupropion efficacy is positively correlated with hydroxybupropion levels, suggesting slow metabolizers may need higher doses to achieve efficacy.1
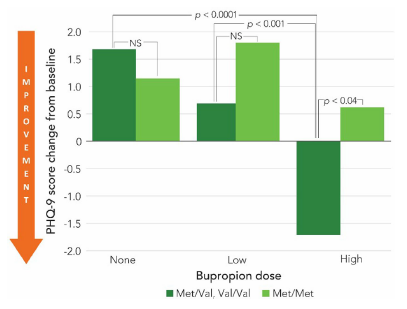
In addition to the CYP2B6 data, a retrospective chart review of patients (n=241) taking bupropion found that individuals carrying a Val allele in their COMT gene saw a greater reduction in PHQ-9 compared to the COMT met/met patients. The results indicated a more pronounced antidepressant response in these patients when receiving high dose bupropion (see Figure 2).2 While the data for COMT genotype is mostly studied in other dopaminergic drugs like amphetamine and methylphenidate for the treatment of ADHD, this preliminary data indicates that these polymorphisms may impact other medications and diagnoses.
Pharmacogenetic Considerations of Mirtazapine (Remeron)
Most pharmacogenetic data collected for mirtazapine center around it’s role as a CYP2D6 substrate. One study found that CYP2D6 ultrarapid metabolizers had a 49% decrease in maximal concentration (Cmax), a 32% decrease in Area Under the Curve (AUC; a measure of total drug exposure), and a 47% increase in clearance of mirtazapine.3
Both CYP3A4 and CYP1A2 genes have also been shown to affect mirtazapine exposure, but data on the contribution of these enzymes on mirtazapine levels has been assessed using CYP inhibitors and inducers. Mirtazapine metabolism has been investigated by the addition of 3A4 inhibitor/inducers like cimetidine and carbamazepine or CYP1A2 inhibitor/inducers like fluvoxamine and cigarette smoking.4-7
Pharmacogenetic Considerations of Trazodone (Desyrel, Oleptro)
Trazodone pharmacokinetics appear to be impacted by changes in CYP3A4 activity, and to a lesser extent CYP2D6. There is some pharmacogenetic data indicating polymorphisms in CYP2D6 may affect the side effect profile (e.g. dizziness) of trazodone.8-10
Pharmacogenetic Considerations of Vilazodone (Viibryd)
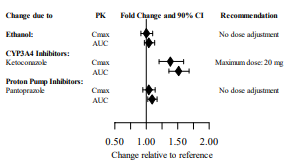
Data from the FDA label for vilazodone indicate that this drug is a substrate of CYP3A4, as ketoconazole increased Cmax and AUC of this drug (see Figure 3). CYP3A4 inducers have been shown to decrease the steady state serum levels of vilazodone by 45%.11
Pharmacogenetic Considerations of Vortioxetine (Trintellix)
Vortioxetine is primarily a CYP2D6 substrate, with a smaller contribution from CYP3A4. When investigating the effects of CYP2D6 polymorphisms in 887 subjects administered vortioxetine, poor metabolizers had 50% of the oral clearance compared to the extensive/normal metabolizers.12,13
The FDA label for vortioxetine specifies that “the maximum recommended dose is 10 mg/day in known CYP2D6 poor metabolizers.” Whenever a drug has specific dosing guidance mentioned in the FDA label, the “Rx” symbol will be placed next to the drug name on the Genomind Professional PGx report (see Figure 4).
n order to make sure this information is quickly accessible by clinicians who use Genomind’s Professional PGx test, the GenMed✔ Pro software will give a brief description of the PGx guidance for this drug if the patient has a significant genotype. The full link to the FDA label (and any other primary source for PGx guidance) is also available within this software.
Are You Ready to Upgrade Your Practice with Genomind?
Get smarter mental health prescribing guidance that’s good for life. Genomind® Professional PGx Express™ is the most advanced and comprehensive mental health pharmacogenetic test available. Get access to 24 genes related to mental health, 130+ medications, 10+ conditions, state-of-the-art tools, and 360 degrees of support today!
References
- Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther. 2012;92(6):771-777.
- Fawver J, Flanagan M, Smith T, Drouin M, Mirro M. The association of COMT genotype with buproprion treatment response in the treatment of major depressive disorder. Brain Behav. 2020;10(7):e01692.
- Brockmöller J, Meineke I, Kirchheiner J. Pharmacokinetics of mirtazapine: enantioselective effects of the CYP2D6 ultra rapid metabolizer genotype and correlation with adverse effects. Clin Pharmacol Ther. 2007;81(5):699-707.
- Sitsen JM, Maris FA, Timmer CJ. Concomitant use of mirtazapine and cimetidine: a drug-drug interaction study in healthy male subjects. Eur J Clin Pharmacol. 2000;56(5):389-394.
- Sitsen J, Maris F, Timmer C. Drug-drug interaction studies with mirtazapine and carbamazepine in healthy male subjects. Eur J Drug Metab Pharmacokinet. 2001;26(1-2):109-121.
- Demers JC, Malone M. Serotonin syndrome induced by fluvoxamine and mirtazapine. Ann Pharmacother. 2001 Oct;35(10):1217-20.
- Scherf-Clavel M, Samanski L, Hommers LG, Deckert J, Menke A, Unterecker S. Analysis of smoking behavior on the pharmacokinetics of antidepressants and antipsychotics: evidence for the role of alternative pathways apart from CYP1A2. Int Clin Psychopharmacol. 2019 Mar;34(2):93-100.
- Prapotnik M, Waschgler R, König P, Moll W, Conca A. Therapeutic drug monitoring of trazodone: are there pharmacokinetic interactions involving citalopram and fluoxetine?. Int J Clin Pharmacol Ther. 2004;42(2):120-124.
- Saiz-Rodríguez M, Belmonte C, Derqui-Fernández N, et al. Pharmacogenetics of trazodone in healthy volunteers: association with pharmacokinetics, pharmacodynamics and safety. Pharmacogenomics. 2017;18(16):1491-1502.
- Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Short-term exposure to low-dose ritonavir impairs clearance and enhances adverse effects of trazodone. J Clin Pharmacol. 2003;43(4):414-422.
- Boinpally R, Gad N, Gupta S, Periclou A. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014;36(11):1638-1649.
- Chen G, Lee R, Højer AM, Buchbjerg JK, Serenko M, Zhao Z. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727-736.
- Areberg J, Petersen KB, Chen G, Naik H. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
Note (title image). Calderone, Julia. “An Expanding Repertoire.” Scientific American, Springer Nature America, Inc, 1 Nov. 2014, www.scientificamerican.com/article/the-rise-of-all-purpose-antidepressants/.

